BMS Tries Again With T Cell Engagers, Turning to Janux to Develop New Tumor-Activated Therapy
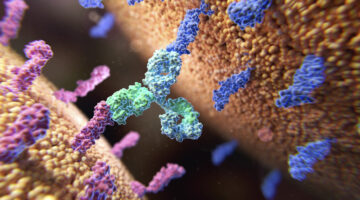
Bristol Myers Squibb is paying $50 million to begin a collaboration with Janux Therapeutics, a company that develops T cell engagers that activate specifically at the site of the tumor. It’s the second big pharma alliance for Janux, which has been working with Merck.